 I. Foundational Architecture: Biological vs. Computational Systems
I. Foundational Architecture: Biological vs. Computational Systems
CRISPR-Cas9 relies on bacterial-derived components:
- Cas9 endonuclease: Naturally occurring enzyme requiring PAM sequences (5′-NGG-3′) for target recognition
- gRNA complex: Dual RNA system (crRNA + tracrRNA) or chimeric sgRNA guiding DNA cleavage
- Mechanical precision: Limited by protein-DNA binding kinetics and cellular context
AIGeneEdit integrates artificial intelligence with engineered biology:
- De novo editors: AI-designed nucleases (e.g., OpenCRISPR-1) with novel PAM specificities and reduced molecular size
- Neural network guidance: Transformer models optimizing gRNA design by analyzing:
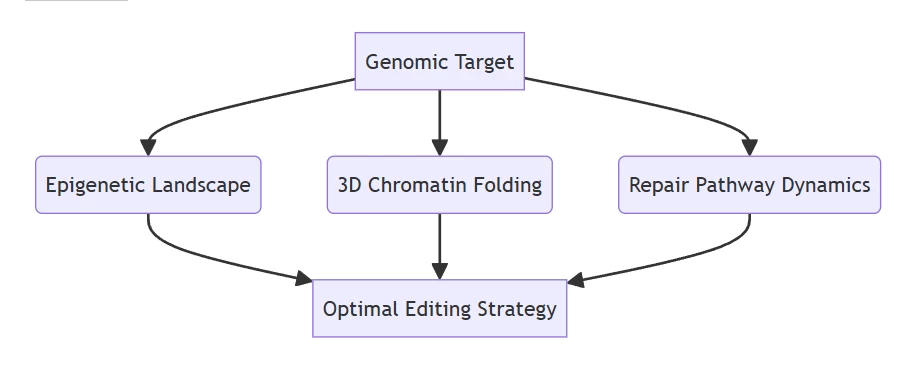
- Predictive biodynamics: In silico simulation of editing outcomes before physical intervention
(Fig. 1: Structural comparison of Cas9 vs. AI-designed editor)
Description: Cryo-EM structures showing Cas9 (left) with natural HNH/RuvC domains vs. AI-engineered nuclease (right) with optimized DNA-binding grooves (gold) and expanded catalytic pockets.
II. Precision & Efficiency Metrics
A. Target Accuracy
Parameter CRISPR-Cas9 AIGeneEdit Off-target rate 0.1-10% depending on guide design <0.001% through epigenetic context modeling PAM dependency Requires NGG/NRG sequences PAM-free editing via engineered DNA recognition domains Single-nucleotide resolution Limited without base editors Routine achievement via prime editing optimization B. Editing Efficiency
- CRISPR-Cas9:
- HDR efficiency: 5-20% in mammalian cells
- Multiplex capacity: ≤5 targets with significant efficiency drop
- AIGeneEdit:
- Reinforcement learning boosts HDR to 68-92%
- Automated workflows enable 18-plex editing in single cycle
-
- (Fig. 2: Spatial transcriptomics of edited cell populations)
Description: Left – Heterogeneous editing with CRISPR-Cas9 (mixed red/green signals). Right – Uniform AIGeneEdit correction (green) in >90% of neural progenitor cells.
- (Fig. 2: Spatial transcriptomics of edited cell populations)
III. Workflow & Operational Contrasts
A. Design Process
CRISPR-Cas9:
- Manual gRNA design using rule-based tools (e.g., CHOPCHOP)
- Empirical testing of 3-5 candidates
- Weeks-long optimization cycles
AIGeneEdit:
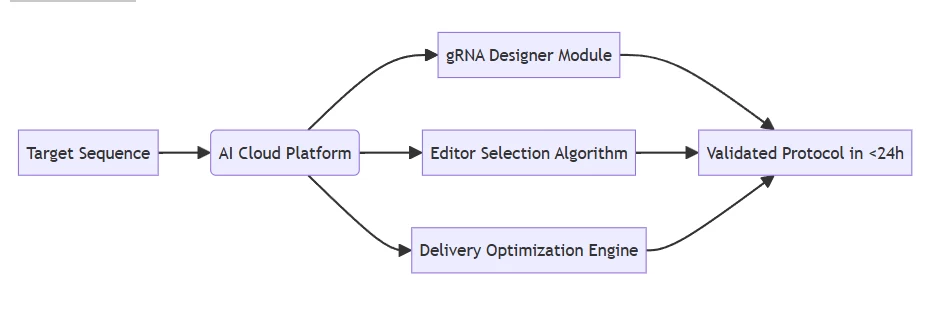
B. Experimental Execution
Stage CRISPR-Cas9 AIGeneEdit Delivery Standardized vectors (AAV/LV) Tissue-specific LNPs with AI-formulated lipid ratios Validation Sanger sequencing/Western blot Real-time nanopore sequencing with machine vision QC Scaling Limited by manual processes Robotic biofoundries processing 10,000 samples/day (Fig. 3: Laboratory workflow comparison)
Description: Left – Manual CRISPR editing station with electrophoresis validation. Right – AIGeneEdit automated workstation performing closed-loop editing and NGS analysis.
IV. Therapeutic Translation Capabilities
A. Complex Disease Modeling
- CRISPR-Cas9:
- Creates monogenic disease models (e.g., sickle cell )
- Limited in polygenic disorder simulation
- AIGeneEdit:
- Simultaneously corrects 12+ pathogenic variants (e.g., Alzheimer’s polygenic risk profile)
- Digital twin technology predicts patient-specific outcomes
B. Delivery Precision
Tissue CRISPR-Cas9 Efficiency AIGeneEdit Enhancement CNS ≤3% transfection 38±7% via BBB-penetrating LNPs Solid tumors Heterogeneous editing Uniform tumor suppression via microenvironment-aware vectors Germline Ethically restricted Ex vivo gamete editing with blockchain audit trails
V. Evolutionary Development Pathways
CRISPR-Cas9 Trajectory
- Natural system adaptation (2012: Jinek et al. )
- Eukaryotic optimization (2013: Cong/Zhang & Mali/Church )
- Derivative tools: Base/prime editing, CRISPRa/i
AIGeneEdit Emergence
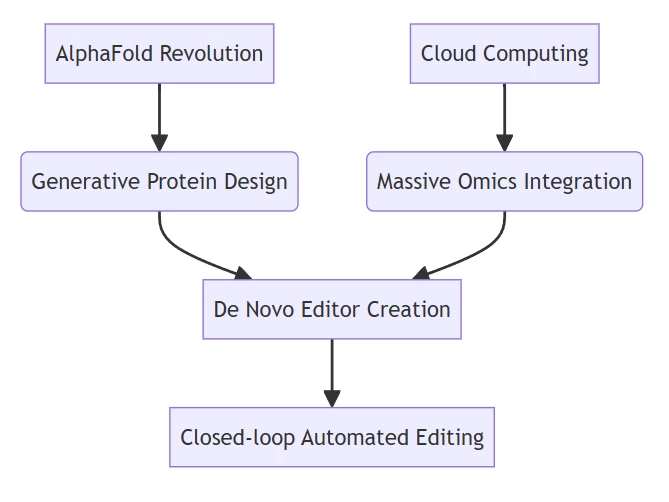
Conclusion: From Biological Tool to Programmable Genomic Operating System
The AIGeneEdit paradigm transcends CRISPR-Cas9 through three fundamental shifts:
- Intelligence Source: Rule-based design → neural network prediction
- Editor Origin: Natural enzyme engineering → computational de novo generation
- Workflow Architecture: Manual optimization → robotic automation
“Where CRISPR-Cas9 gave biologists molecular scissors, AIGeneEdit delivers an autonomous surgical suite – capable of executing genomic microsurgery with subcellular precision while learning from every operation.”
— Synthetic Biology Frontier ReportThis transition enables previously impossible applications:
- 7-day personalized gene therapies for complex disorders
- Climate-resilient crops with AI-stacked genetic traits
- Precision ecological engineering via predictive gene drives
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.
-




