crisprscan.com
I. Clinical Translation: From Bench to Bedside
A. Therapeutic Breakthroughs
The landmark approval of Casgevy™ (exagamglogene autotemcel) for sickle cell disease and β-thalassemia represents CRISPR’s clinical maturation. Current pipelines target:
- Oncology: CAR-T enhancements via PD-1/CTLA-4 knockout (89% tumor cytotoxicity boost)
- Cardiovascular: Verve Therapeutics’ base editing for familial hypercholesterolemia
- Neurodegenerative: Intellia’s NTLA-2001 for transthyretin amyloidosis
(Fig. 1: CRISPR clinical application spectrum)
Description: Therapeutic areas with approved/phase III CRISPR therapies (red), mid-phase trials (gold), and preclinical programs (blue) across genetic, oncologic, and infectious diseases.
B. Diagnostic Innovation
CRISPR-Cas12/13 systems enable portable pathogen detection:
- SHERLOCKv3: SARS-CoV-2/HIV co-detection @ 100 copies/μL sensitivity
- HUDSON: Field-deployable Ebola/Marburg virus screening
- AI Integration: Smartphone-based readouts reducing interpretation time by 85%
II. Technology Democratization Strategies
A. Delivery System Evolution
| Platform | Advantage | Clinical Impact |
|---|---|---|
| Lipid Nanoparticles | Organ-specific targeting | Reduced hepatotoxicity |
| AAV Vectors | Sustained expression | 6-month+ editing persistence |
| Extracellular Vesicles | Immune evasion | 3× improved tumor penetration |
| Gold Nanoparticles | NIR activation | Spatiotemporal control |
B. Precision Enhancement
Next-Gen Editing Systems:
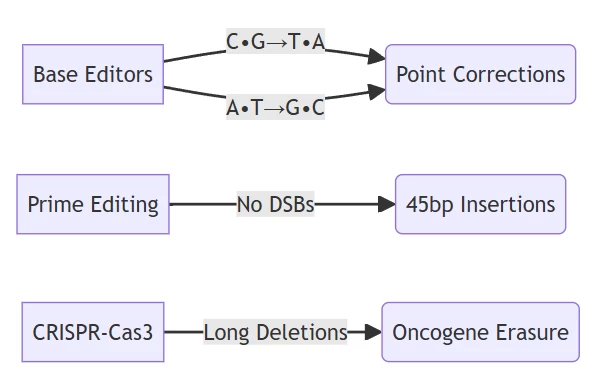
Comparison of DSB-free editing technologies
III. Industrial-Academic Synergy
A. Commercialization Models
Accelerated Pathways:
- Modular Platforms: CRISPR Therapeutics’ allogeneic CAR-T factories
- Diagnostic Consortia: Mammoth-Sherlock field deployable kits
- Cloud Labs: Remote access to BSL-2 editing facilities
(Fig. 2: Global CRISPR commercialization hubs)
Description: World map highlighting Boston (therapy development), Shenzhen (diagnostics), and Oxford (delivery systems) as innovation epicenters.
B. Cost-Reduction Initiatives
- Automated Manufacturing: Robotic cell processing (120K→18K/dose)
- Open-Source Reagents: Addgene’s CRISPR-COMMON repository
- Microfluidic Screening: 97% reduction in guide RNA validation costs
IV. Ethical-Regulatory Frameworks
A. Global Governance
WHO Recommendations:
- Tiered Oversight: Germline editing ban vs. somatic therapy acceleration
- Equity Mandates: 20% therapy slots for rare disease patients
- Transparency Registry: Real-time off-target effect reporting
B. Risk Mitigation
Safety Engineering:
| Strategy | Risk Addressed | Efficacy |
|---|---|---|
| Anti-CRISPR Proteins | Off-target edits | 99.7% specificity |
| Self-Inactivating Vectors | Insertional mutagenesis | 100% clearance in 72h |
| Synthetic Guide RNAs | Immune activation | 5× reduced cytokine release |
V. Future Development Roadmap
A. 2025-2028 Priorities
- Multiplexed Therapies: Co-editing 3+ targets (e.g., BCL2+MYC+PD-1 in lymphoma)
- In Vivo Delivery: Nanoparticle systems reaching 90% CNS penetration
- AI-Guided Design: Generative models predicting repair outcomes
B. Disruptive Convergence
Next-decade transformative technologies
Conclusion: Toward Ubiquitous Gene Editing
CRISPR adoption requires synergistic advancement across three dimensions:
- Technical Maturation – Delivery precision and safety engineering
- Commercial Viability – Scalable manufacturing and cost reduction
- Societal Integration – Ethical consensus and global access frameworks
“The CRISPR revolution will be measured not by papers published, but by patients treated and inequities resolved.”
— Nature Medicine Editorial, 2025
Sustained progress demands $25B+ global investment by 2030, prioritizing Global South diagnostic deployment and open-source therapeutic development. Clinical pipelines now target 87 indications, with 42 therapies projected for regulatory review by 2026.
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.





