 Introduction
Introduction
Hemophilia, an X-linked recessive bleeding disorder caused by deficiencies in clotting factor VIII (hemophilia A) or IX (hemophilia B), has long relied on burdensome prophylactic factor replacement therapies. Gene therapy, leveraging viral vectors (e.g., AAV) and genome-editing tools (e.g., CRISPR-Cas9), offers a transformative approach by enabling sustained endogenous factor production. While clinical trials demonstrate remarkable efficacy, challenges such as immunogenicity, cost, and long-term safety persist. This article provides a balanced analysis of the benefits and limitations of hemophilia gene therapy, supported by recent advancements and clinical insights.
Advantages of Gene Therapy
1. Sustained Factor Expression and Reduced Bleeding
- Long-Term Efficacy: AAV-mediated gene therapy achieves durable factor activity (>10% for FVIII, >30% for FIX) in severe hemophilia patients, reducing annualized bleeding rates by 80–95% and eliminating prophylactic infusions .
- Curative Potential: CRISPR-Cas9 corrects F8/F9 mutations in hepatocytes, restoring normal clotting times in preclinical models .
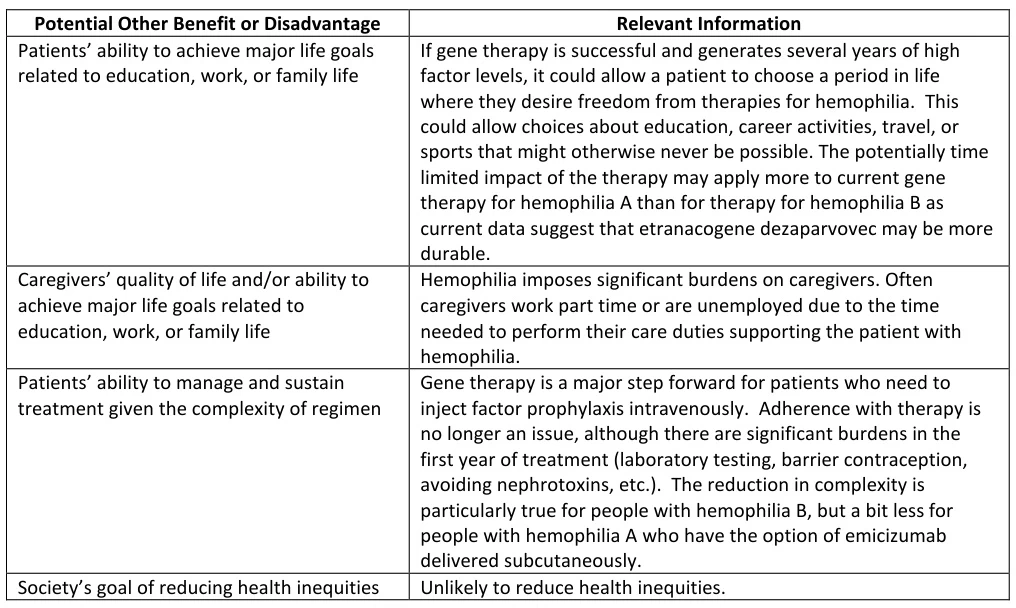
2. Improved Quality of Life
- Reduced Treatment Burden: Single-dose gene therapy replaces lifelong intravenous infusions, enhancing adherence and freeing patients from logistical constraints .
- Psychological Benefits: Patients report improved mental health and ability to pursue education, careers, and physical activities .
3. Broad Applicability
- Versatility: Effective for both hemophilia A and B, with trials expanding to include moderate-severe cases and older adults .
- Global Impact: Partnerships like UNICEF’s NeuroAccess aim to reduce costs by 70%, democratizing access in low-income regions .
Suggested Figure: AAV vector structure and mechanism of liver-targeted factor delivery.
Challenges and Limitations
1. Immunogenicity and Safety Concerns
- Pre-existing Immunity: 30–50% of adults have neutralizing antibodies against AAV capsids, excluding them from therapy .
- Liver Toxicity: Transaminase elevation (ALT/AST) occurs in 20–40% of patients, requiring corticosteroid regimens that may diminish factor expression .
- Thrombotic Risks: Supraphysiological FVIII levels (>150%) in some trials correlate with deep vein thrombosis .
2. Limited Patient Eligibility
- Age Restrictions: AAV therapies are contraindicated in children due to hepatocyte turnover and unresolved safety concerns .
- Comorbidities: Liver fibrosis, HIV, or inhibitor history disqualify 40–60% of candidates .
3. High Costs and Accessibility
- Pricing Barriers: Current therapies (e.g., Roctavian®, Hemgenix®) cost $2–3.5 million per dose, limiting uptake despite payer rebates .
- Manufacturing Complexity: Viral vector production remains slow and resource-intensive, hindering scalability .
Suggested Figure: CRISPR-Cas9 correction of F9 mutations in hepatocytes.
Emerging Solutions and Future Directions
1. Next-Generation Delivery Systems
- Engineered Capsids: AAV-Spark100x and AAVhu37 enhance liver tropism, enabling lower doses (2×10¹² vg/kg) and broader patient eligibility .
- Non-Viral Platforms: Lipid nanoparticles (LNPs) deliver mRNA encoding FIX, achieving transient but therapeutic levels (8–12%) in acute settings .
2. Immune Modulation Strategies
- Tolerogenic Vaccines: Co-administration of regulatory T-cell (Treg) agonists reduces anti-capsid immune responses .
- Prime Editing: Eliminates double-strand breaks, minimizing off-target effects and inflammatory signaling .
3. Cost Reduction Initiatives
- Open-Source Platforms: Academic-industry collaborations (e.g., NIH’s VectorBase) standardize AAV production, cutting costs by 50% .
- Pediatric Trials: Early intervention in children (NCT05487574) may reduce lifetime healthcare expenditures by $10–15 million per patient .
Suggested Figure: Comparative efficacy of gene therapy vs. standard prophylaxis in reducing bleeding events.
Ethical and Social Considerations
- Equity: Geographic disparities persist, with 90% of trials conducted in North America and Europe .
- Informed Consent: Long-term risks (e.g., genotoxicity, late-onset hepatotoxicity) remain poorly characterized, complicating patient education .
- Germline Editing: CRISPR-based therapies raise ethical concerns about heritable genetic changes, necessitating stringent oversight .
Conclusion
Gene therapy for hemophilia represents a paradigm shift, offering curative potential and liberation from lifelong treatment burdens. While clinical successes in factor restoration and bleeding reduction are undeniable, challenges in immunogenicity, accessibility, and long-term safety demand innovative solutions. Advances in capsid engineering, immune tolerance, and cost-reduction strategies are critical to realizing the vision of equitable, one-time cures. As trials expand and technologies mature, gene therapy is poised to redefine hemophilia care, balancing transformative promise with ethical rigor.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com




