 I. Fundamental Genomic Definitions
I. Fundamental Genomic Definitions
Positive-Sense RNA (+ssRNA)
- Molecular Identity: Functions as immediate messenger RNA (mRNA) upon host cell entry, with its nucleotide sequence directly readable by host ribosomes for instantaneous protein synthesis .
- Key Attribute: Genome is infectious in purified form, capable of initiating viral replication without viral proteins (e.g., poliovirus RNA) .
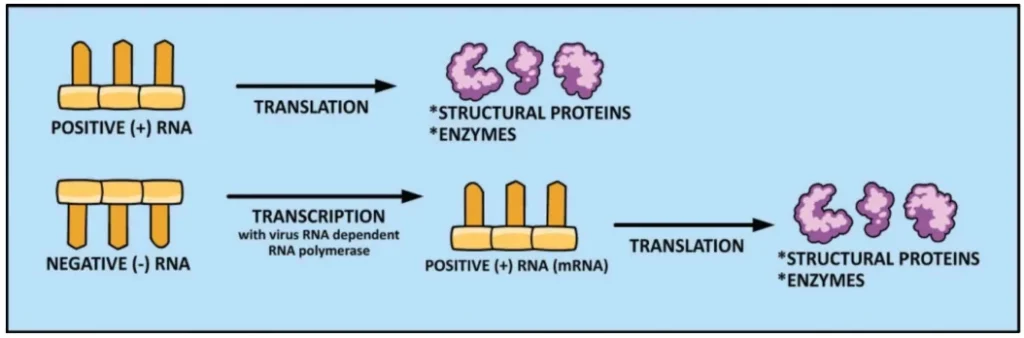
Negative-Sense RNA (-ssRNA)
- Molecular Identity: Complementary to mRNA; cannot initiate translation. Requires virion-packaged RNA-dependent RNA polymerase (RdRp) to synthesize +ssRNA intermediates .
- Key Attribute: Non-infectious as purified RNA due to absolute RdRp dependency .
(Fig. 1: Genomic Polarity Illustrated)
Description: Left: +ssRNA (blue) binding directly to ribosome (grey) for translation. Right: -ssRNA (red) requiring RdRp (yellow) to generate translatable +ssRNA.
II. Replication Mechanisms: A Comparative Workflow
Positive-Sense Viral Cycle
- Immediate Translation: Genomic +ssRNA → viral polyprotein → RdRp production .
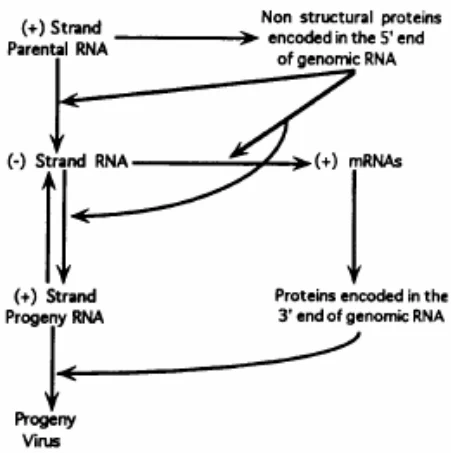
- Replication Complex Assembly: RdRp synthesizes complementary -ssRNA → forms dsRNA intermediate .
- Asymmetric Amplification: -ssRNA template generates 10-100x more +ssRNA progeny .
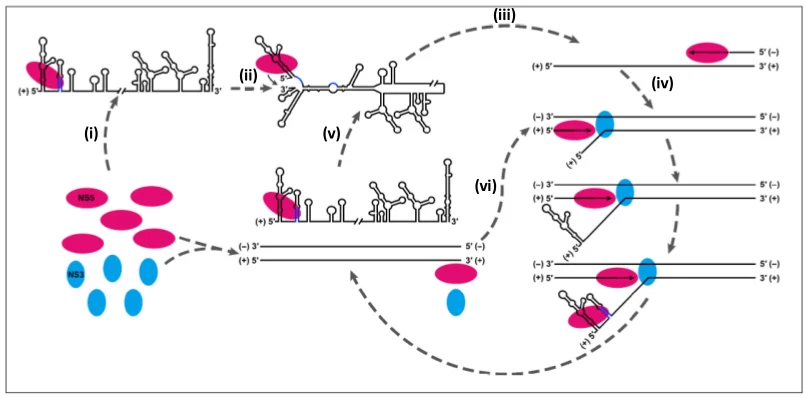
- Progeny Fate: New +ssRNA → mRNA translation or encapsidated genomes .
Negative-Sense Viral Cycle
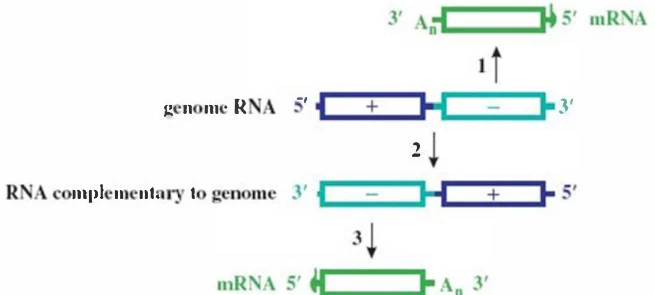
- RdRp Priming: Virion-carried RdRp transcribes -ssRNA → +ssRNA mRNAs .
- Replication Switch: +ssRNA → antigenome (-ssRNA) → progeny genomes .
- Genome Protection: RNA-Nucleoprotein (RNP) complexes prevent host immune detection .
(Fig. 2: Replication Cycles Compared)
Description: Top: +ssRNA virus cycle showing direct translation and asymmetric replication. Bottom: -ssRNA cycle emphasizing RNP complexes and RdRp-driven transcription.
III. Structural & Functional Consequences
| Characteristic | +ssRNA Viruses | -ssRNA Viruses |
|---|---|---|
| RdRp Packaging | Synthesized de novo in host | Pre-packaged in virion |
| Genome Architecture | Often non-segmented | Frequently segmented (e.g., influenza) |
| Mutation Rate | High (no proofreading; e.g., Coronaviridae) | Lower (RNP protection) |
| Host Defense Evasion | Membrane-bound replication complexes | Nuclear/cytoplasmic RNP “factories” |
| Clinical Examples | SARS-CoV-2, Hepatitis C, Zika | Influenza, Ebola, Rabies |
(Fig. 3: Replication Complex Ultrastructure)
Description: 3D cutaway of +ssRNA replicase (green) bound to endoplasmic reticulum. -ssRNA RNP complex (orange) with N-protein (purple) coating RNA.
IV. Evolutionary Strategies & Clinical Impact
A. Therapeutic Targeting
- +ssRNA Vulnerabilities:
- RdRp inhibitors (Remdesivir)
- Protease blockers (Nirmatrelvir)
- -ssRNA Vulnerabilities:
- Nucleoprotein disruptors
- RdRp allosteric inhibitors (Baloxavir)
B. Pandemic Risks
- +ssRNA Threats: Rapid evolution enables zoonotic jumps (e.g., COVID-19 → 7M+ deaths) .
- -ssRNA Threats: Reassortment in segmented viruses (e.g., influenza pandemics) .
V. Diagnostic & Biotechnological Applications
A. Detection Methods
| Viral Class | Key Diagnostic Target | Technology |
|---|---|---|
| +ssRNA | Genomic RNA (direct detection) | RT-PCR |
| -ssRNA | Early-transcribed mRNA | NASBA/TMA amplification |
B. Synthetic Biology Platforms
- +ssRNA Tools: Self-amplifying mRNA vaccines (Moderna, Pfizer) .
- -ssRNA Engineering: RNP delivery for gene therapy .
VI. Unresolved Scientific Questions
- Evolutionary Paradox: Why do +ssRNA viruses dominate plant pathogens (80%), while -ssRNA target vertebrates?
- Error-Correction Mechanisms: How do RdRp fidelity factors differ between classes?
- Compartmentalization: Why do most -ssRNA viruses replicate in the cytoplasm, except Orthomyxoviridae?
“Genomic polarity dictates viral life history: +ssRNA prioritizes explosive adaptability, while -ssRNA evolves through genomic stability via structural innovation.”
— Nature Reviews Microbiology, 2024
Data sourced from publicly available references. For collaboration inquiries, contact: chuanchuan810@gmail.com.




