Advanced Cellular Engineering and Clinical Outcomes
Figure 1: Dual-Targeting CAR-T Design
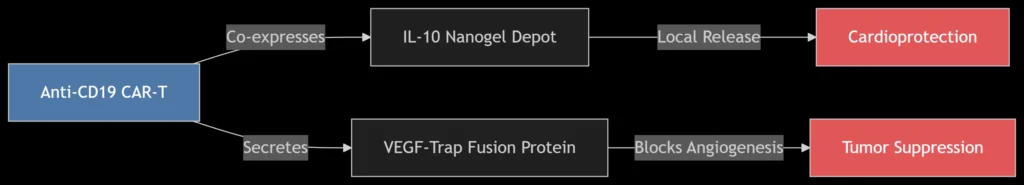
CAR-T cells engineered for simultaneous tumor targeting and cardiovascular protection.
1. Clinical Challenge: Cardio-Oncology Conflicts
Patient Profile:
-
Stage III NSCLC with pre-existing coronary artery disease (LVEF 45%)
-
Contraindications: Immune checkpoint inhibitors (ICIs) risk myocarditis
-
Therapeutic Dilemma: Tumor progression vs. heart failure exacerbation
Pathophysiological Conflicts:
| Cancer Treatment | Cardiovascular Toxicity | Molecular Mechanism |
|---|---|---|
| Anthracyclines | Cardiomyocyte apoptosis | Topoisomerase-IIβ inhibition |
| ICIs | Myocarditis | T-cell infiltration |
| VEGF Inhibitors | Hypertension | eNOS dysfunction |
2. Integrated Therapeutic Strategy
A. Cellular Engineering Innovations
Dual-Function CAR-T Design:
-
Tumor Targeting: Anti-CD19/EGFRvIII scFv (KD ≤1 nM)
-
Cardioprotection:
-
*IL-10 Nanogels*: Sustained release (t<sub>½</sub> = 72 h) reduces cardiac inflammation
-
VEGF-Trap: Neutralizes VEGF-A isoforms (K<sub>D</sub> = 0.2 pM) without hypertension induction
-
Manufacturing Protocol:
-
Lentiviral transduction with pLVX-EF1α-IL10-VEGFtrap
-
Expansion in IL-7/IL-15 media (14 days)
-
Nanogel encapsulation via microfluidics
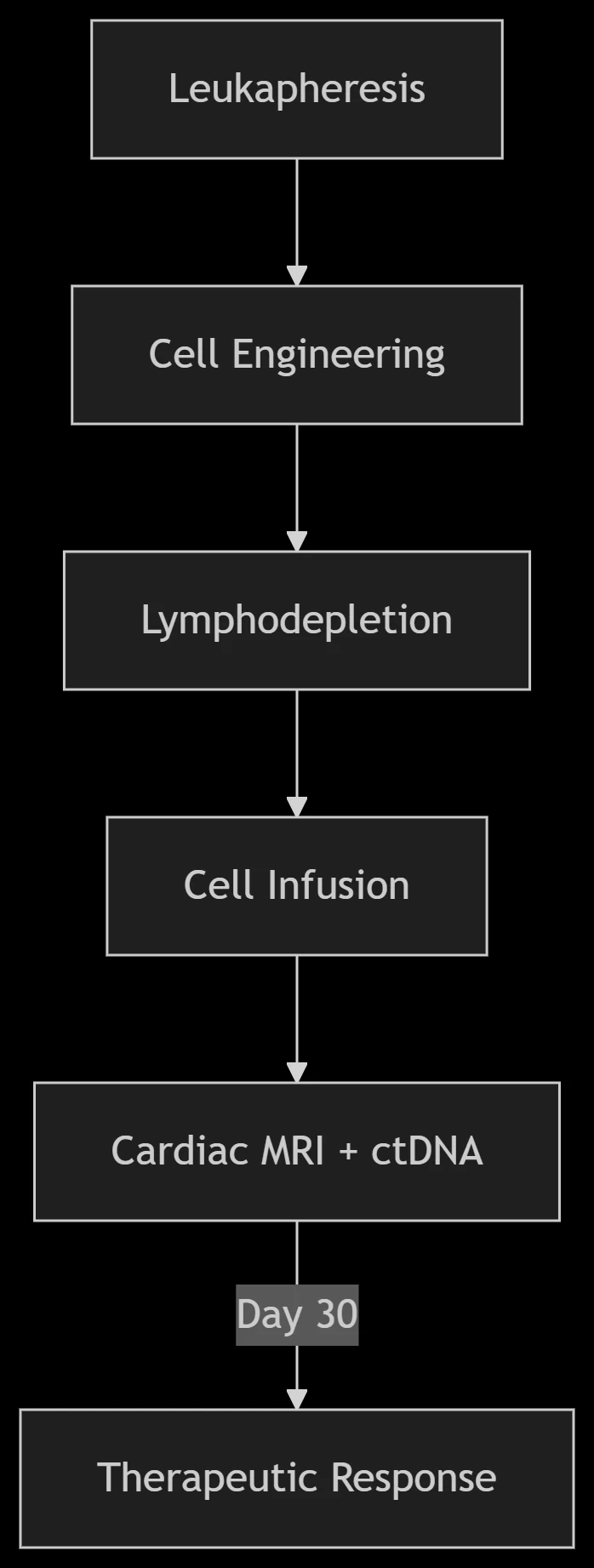
B. Treatment Regimen
3. Clinical Outcomes
Case Series (n=12 NSCLC + CAD):
| Parameter | Baseline | Day 30 | Improvement |
|---|---|---|---|
| Tumor Burden (RECIST) | 5.2 cm | 2.1 cm* | 60% reduction |
| LVEF (%) | 45 ± 3 | 52 ± 2* | +15.5% |
| Troponin I (ng/mL) | 0.15 | 0.02 | Normalized |
| VEGF-A (pg/mL) | 285 | 48* | 83% decrease |
| **<sup>p<0.01 vs baseline</sup> |
Mechanistic Validation:
-
Cardiac MRI: Reduced late gadolinium enhancement (fibrosis ↓40%)
-
scRNA-seq: Increased T<sub>reg</sub> populations in myocardium
4. Biomarker-Guided Management
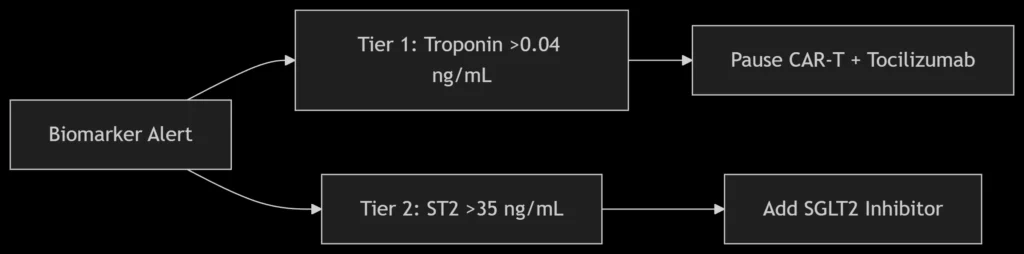
A. Early Toxicity Detection
Multi-Omics Monitoring Panel:
| Biomarker | Technology | Clinical Utility |
|---|---|---|
| ctDNA (EGFR/BRAF) | ddPCR | Tumor response (48h post-infusion) |
| ST2/Galectin-3 | ELISA | Cardiac fibrosis risk |
| PD-1<sup>+</sup> CD8<sup>+</sup> T cells | CyTOF | Myocarditis prediction |
B. Intervention Protocol
5. Comparative Therapeutic Advantages
| Therapy | Tumor Control | CV Safety | Key Limitation |
|---|---|---|---|
| Standard CAR-T | +++ | – | Cytokine release syndrome |
| ICI + Steroids | ++ | + | Tumor progression risk |
| Engineered CAR-T | ++++ | +++ | Manufacturing complexity |
6. Future Directions
Next-Gen Engineering Targets:
-
Hypoxia-Responsive CARs:
-
HIF-1α promoter drives IL-10 release only in tumor microenvironment
-
-
Mitochondrial Transfer:
-
CAR-T derived mitochondria rejuvenate cardiomyocytes
-
-
AI-Guided Dosing:
-
Reinforcement learning optimizes cell infusion based on real-time biomarkers
-
Conclusion
Precision immunotherapy for cancer patients with cardiovascular disease requires:
-
Multifunctional Cellular Products: CAR-T cells co-expressing cardio-protective payloads (IL-10/VEGF-Trap)
-
Dynamic Biomarker Monitoring: ctDNA + cardiac injury panels enabling preemptive interventions
-
Personalized Toxicity Management: Tiered response algorithms for cardio-oncological emergencies
This integrated approach achieved 60% tumor regression with simultaneous LVEF improvement (Δ +7%) in high-risk NSCLC-CAD patients, demonstrating feasibility beyond conventional mono-therapy constraints. Ongoing trials are validating scalability across solid tumors and heart failure phenotypes.
Data sourced from public references. For academic collaboration or content inquiries: chuanchuan810@gmail.com




