Precision in Translation Termination and Genome Annotation
1. Defining CDS and Stop Codon Roles
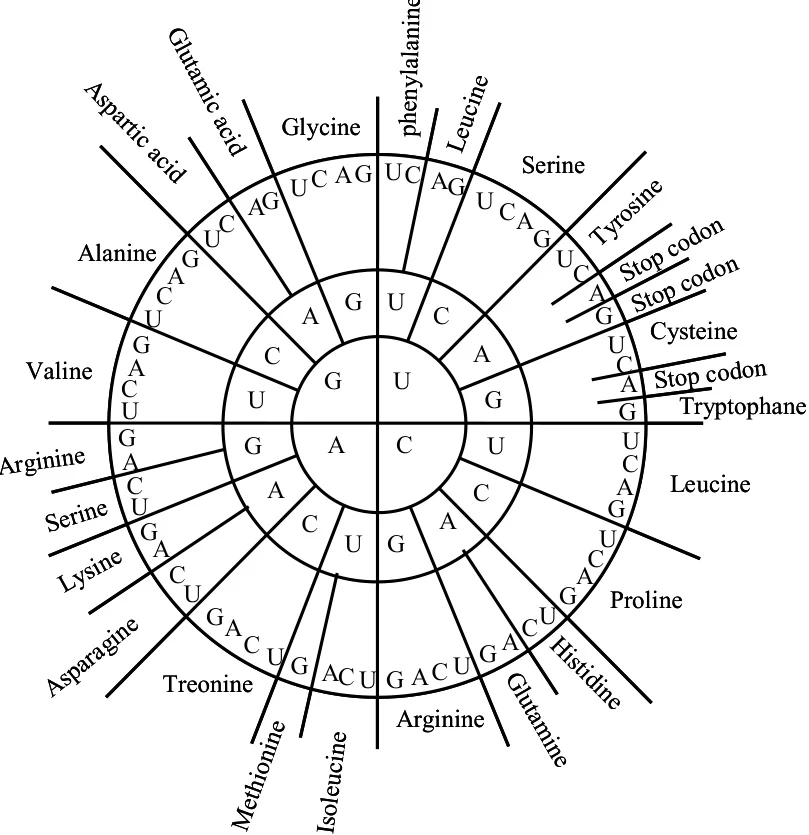
A coding sequence (CDS) is the region of a gene or mRNA that specifies the amino acid sequence of a protein. CDS boundaries are demarcated by a start codon (typically AUG, encoding methionine) and a stop codon (UAA, UAG, or UGA), which signals translation termination. Stop codons are critical for ensuring accurate protein synthesis, preventing aberrantly elongated polypeptides that could disrupt cellular function.
2. Stop Codon Usage in CDS Annotation
A. Universal Genetic Code Framework
In the standard genetic code, UAA, UAG, and UGA are recognized as termination signals. These codons do not encode amino acids but recruit release factors (e.g., eRF1 in eukaryotes) to dissociate ribosomal subunits and release nascent polypeptides.
- CDS Integrity: Accurate identification of stop codons is essential for defining the 3′ end of CDS. Misannotation (e.g., failing to detect a stop codon) can lead to incorrect protein predictions, including frameshifts or pseudo-gene assignments.
- Bioinformatics Tools: Algorithms like ORF finders and GeneMark scan genomic sequences for start/stop codon pairs to predict CDS regions. For example, M. signifera mitochondrial genome annotation revealed conserved TAA (UAA in RNA) as the primary stop codon across most genes.
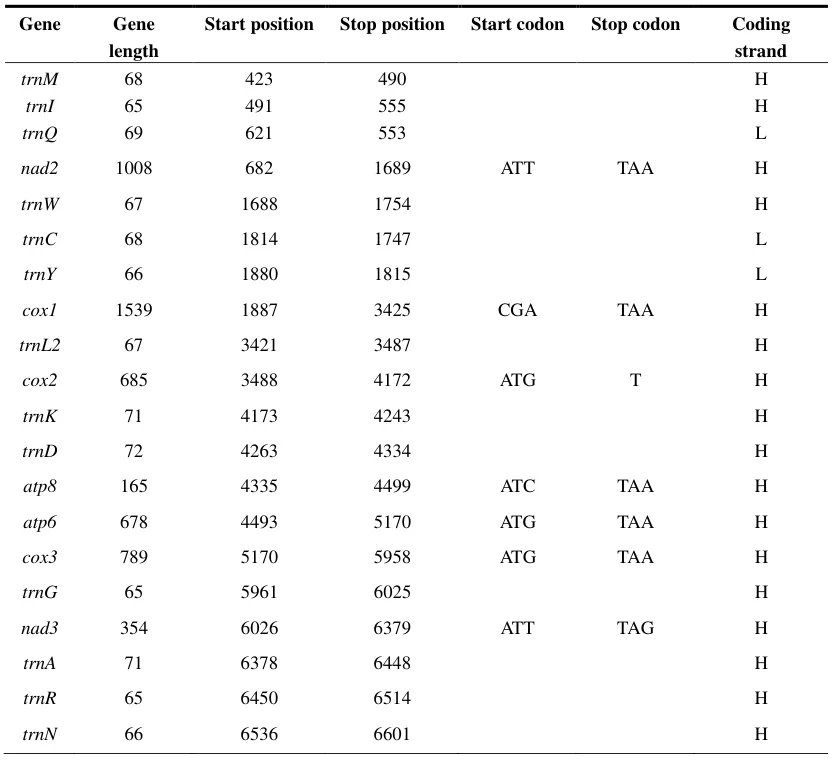
Image suggestion: Workflow diagram of CDS prediction, highlighting start/stop codon identification and open reading frame (ORF) analysis.
3. Stop Codon Distribution and Evolutionary Constraints
A. Species-Specific Preferences
- Prokaryotes: UAA (TAA in DNA) is the most frequent stop codon, favored in highly expressed genes due to its efficiency in translation termination. For instance, in E. coli K-12, UAA accounts for 63% of stop codons, while UAG (7.4%) is rare.
- Eukaryotes: UGA is often dual-functional, encoding selenocysteine in SECIS-containing mRNAs (e.g., human selenoproteins).
- Mitochondria: Vertebrate mitochondria repurpose AGA and AGG (normally arginine codons) as additional stop signals, reflecting evolutionary adaptation to reduced genome complexity.
B. GC Content Influence
In GC-rich genomes, UGA usage increases due to its higher GC content (UGA in RNA corresponds to TGA in DNA, which is GC-neutral). Conversely, AT-rich genomes favor UAA (TAA in DNA).
Image suggestion: Heatmap comparing stop codon frequencies across species with varying GC content.
4. Challenges in CDS Annotation: Ambiguity and Recoding
A. Context-Dependent Stop Codons
Some organisms exhibit ambiguous stop codons, where UAA, UAG, or UGA encode amino acids depending on sequence context:
- Ciliates: In Condylostoma magnum, all three codons can act as stop signals or encode amino acids. Stop codon usage is enriched near transcript termini, minimizing premature termination.
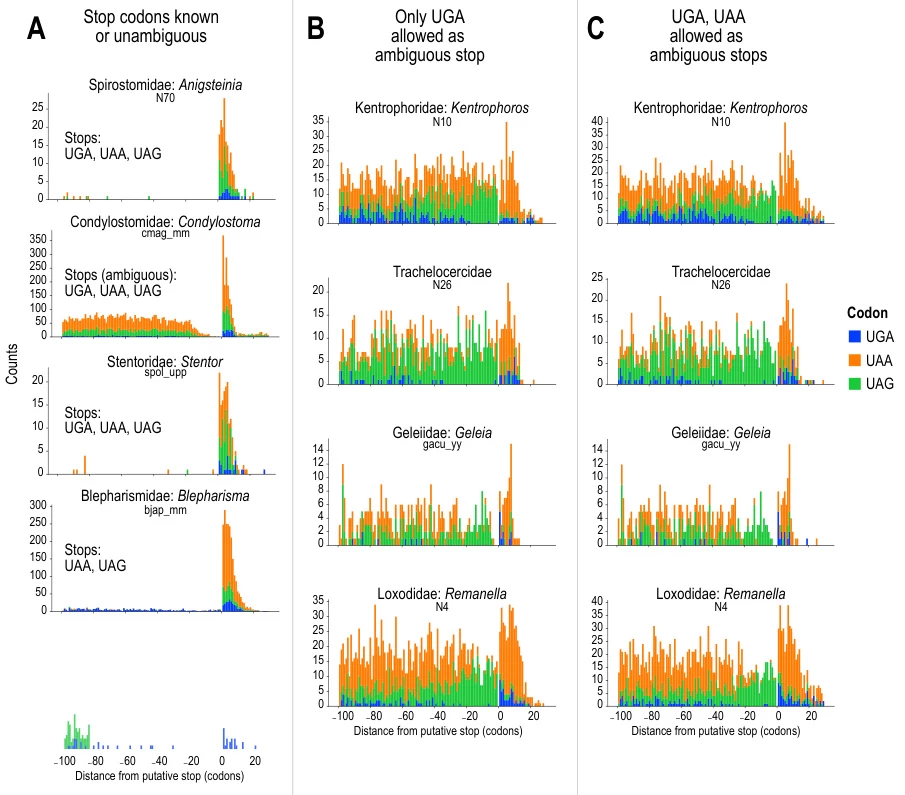
- Kinetoplastids: Blastocrithidia spp. reassign UAG and UAA to glutamine and UGA to tryptophan, requiring specialized tRNA and eRF1 modifications for accurate CDS annotation.
B. Recoded Genomes
Engineered organisms like the genomically recoded organism (GRO) replace all natural stop codons with UAA (TAA in DNA). This frees UAG and UGA for non-canonical amino acid incorporation, expanding CDS flexibility while maintaining precise termination.
Image suggestion: Schematic of a recoded genome, showing TAA homogenization and repurposed UAG/UGA for synthetic biology applications.
5. Functional and Therapeutic Implications
A. Nonsense Mutations and Readthrough Therapies
Premature stop codons (PTCs) truncate proteins, causing diseases like cystic fibrosis. Small molecules (e.g., ataluren) promote ribosomal readthrough of PTCs, restoring partial protein function. Accurate CDS annotation is critical for identifying PTCs and designing therapies.
B. CRISPR-Cas9 Applications
- Gene Knockout: Introducing stop codons into CDS disrupts gene function (e.g., PD-1 knockout in CAR-T cells).
- Exon Skipping: sgRNAs targeting splice sites can exclude exons containing PTCs, restoring functional protein production in disorders like Duchenne muscular dystrophy.
Image suggestion: CRISPR-Cas9 editing workflow, showing stop codon insertion to disrupt a target gene.
6. Emerging Frontiers in CDS-Stop Codon Biology
- Synthetic Genomics: Recoded organisms with unified stop codons (e.g., GRO) enable multiplexed non-standard amino acid incorporation, advancing synthetic protein engineering.
- AI-Driven Annotation: Machine learning models predict stop codon impacts on CDS integrity, improving genome annotation in non-model organisms.
Conclusion
Stop codons (UAA, UAG, UGA) are indispensable for defining CDS boundaries and ensuring translational fidelity. Their usage varies across species due to evolutionary pressures, GC content, and functional plasticity (e.g., selenocysteine encoding). Advances in genome engineering and bioinformatics continue to refine CDS annotation, addressing challenges posed by ambiguous or recoded stop signals. As synthetic biology and precision medicine evolve, understanding stop codon-CDS relationships will remain central to decoding genomic complexity.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com




