Architectural Blueprint for Precision Genome Editing
I. Core Structural Components
sgRNA is a synthetic, single-stranded RNA molecule engineered to guide the CRISPR-Cas9 system to specific genomic loci. Its architecture integrates functional domains from two natural RNAs—crRNA (CRISPR RNA) and tracrRNA (trans-activating crRNA)—into a unified scaffold optimized for target recognition and Cas9 binding.
1. Target-Specific crRNA Module
- Protospacer: A 20-nucleotide (nt) sequence complementary to the target DNA adjacent to the protospacer adjacent motif (PAM) (e.g., 5′-NGG-3′ for Streptococcus pyogenes Cas9). This region ensures sequence-specific hybridization via Watson-Crick base pairing .
- PAM Proximity: The protospacer is positioned 3–8 nt upstream of the PAM, enabling Cas9 nuclease activation .
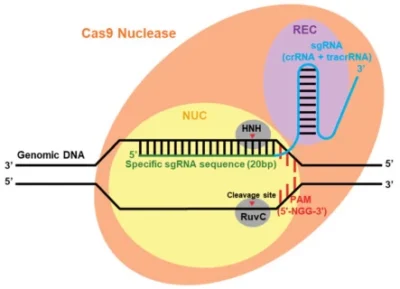
2. Cas9-Binding tracrRNA Scaffold
- Stem-Loop Structures: Three conserved hairpins (stem-loop 1–3) stabilize interactions with Cas9’s REC lobe (recognition lobe). These motifs include:
- Stem-Loop 1: Anchors the sgRNA-Cas9 complex.
- Stem-Loop 2: Facilitates R-loop formation during DNA hybridization.
- Stem-Loop 3: Enhances nuclease activation .
- Nexus Region: A central junction connecting stem-loops 1 and 2, critical for maintaining structural integrity .
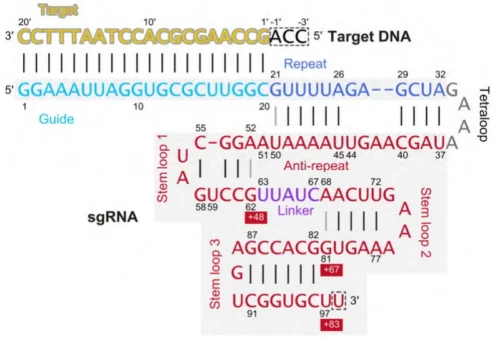
Image suggestion: Atomic-resolution model of sgRNA bound to Cas9, highlighting protospacer-PAM pairing and stem-loop interactions.
II. Secondary and Tertiary Structural Dynamics
sgRNA adopts a T-shaped 3D conformation that balances rigidity for Cas9 binding with flexibility for target scanning:
1. RNA-DNA Hybrid (R-Loop)
- Formation: The protospacer pairs with the DNA target strand, displacing the non-target strand to form an R-loop. This structure positions Cas9’s nuclease domains (HNH and RuvC) for double-strand break induction .
- Conformational Changes: sgRNA binding triggers a structural rearrangement in Cas9, transitioning it from an inactive state to a DNA recognition-competent state .
2. Anti-Repeat and Linker Regions
- Anti-Repeat: A conserved sequence complementary to the tracrRNA’s repeat region, ensuring proper crRNA-tracrRNA hybridization in natural systems. In sgRNA, this region is embedded within the scaffold .
- Linker Sequences: Flexible regions between stem-loops allow adaptive folding during Cas9 activation .
Image suggestion: Dynamic R-loop formation visualized via cryo-EM, showing sgRNA-DNA hybridization and Cas9 conformational shifts.
III. Functional Modules and Engineering Strategies
sgRNA’s design can be modularly optimized to enhance specificity, stability, and versatility:
1. Chemical Modifications
- 2′-O-Methylation: Protects sgRNA from RNase degradation, particularly in mammalian cells .
- Phosphorothioate Backbone: Increases resistance to exonuclease activity, improving in vivo stability .
2. Extended sgRNA Designs
- Truncated Variants: Shorter sgRNAs (e.g., +48 or +54 nt) reduce off-target effects while retaining activity .

- Scaffold Engineering: Modified stem-loop sequences (e.g., MS2 or PP7 aptamers) enable recruitment of effector proteins (e.g., transcriptional activators/repressors) .
3. Sequence Optimization Guidelines
- GC Content: Maintain 30–70% to minimize secondary structures and ensure efficient hybridization .
- Seed Sequence: The 8–12 nt at the 3′ end of the protospacer (closest to the PAM) are critical for specificity; mismatches here significantly reduce off-target effects .
Image suggestion: Heatmap comparing on-target efficiency vs. off-target risk for sgRNAs with varying GC content and seed sequences.
IV. Structural Compatibility with Diverse Cas Proteins
sgRNA scaffolds are tailored to specific Cas9 orthologs or engineered variants:
| Cas Protein | sgRNA Compatibility |
|---|---|
| SpCas9 (S. pyogenes) | 100-nt scaffold with stem-loops 1–3; requires 5′-NGG-3′ PAM . |
| SaCas9 (S. aureus) | Shorter scaffold (∼80 nt); recognizes 5′-NNGRRT-3′ PAM . |
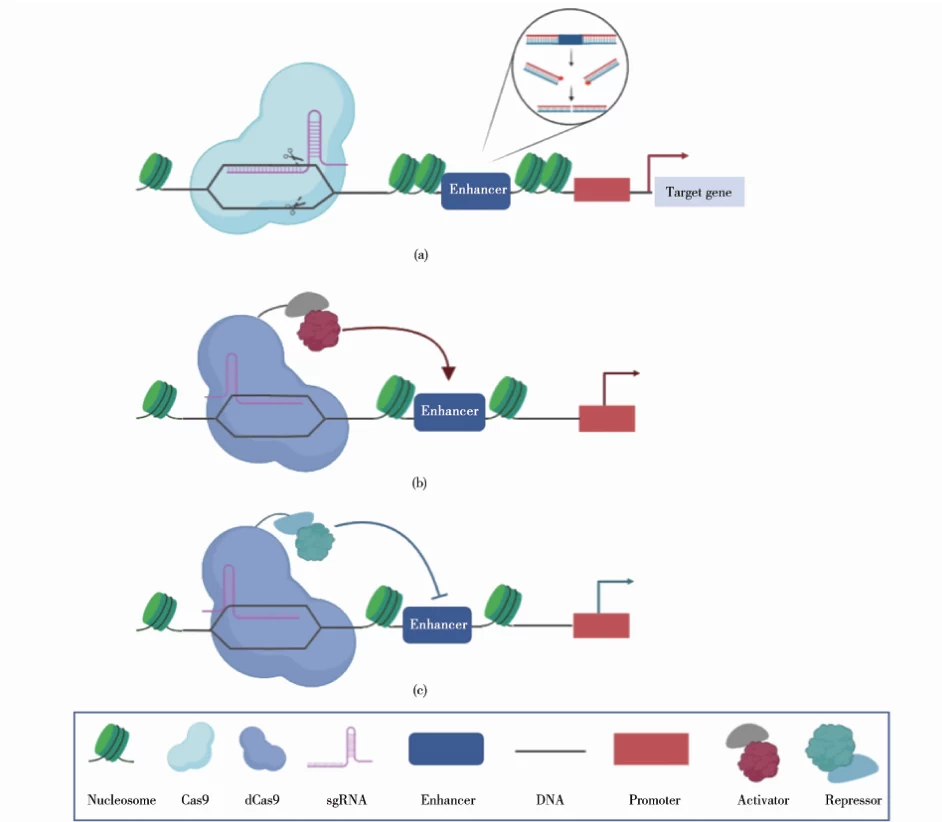
| dCas9 (nuclease-dead) | Retains sgRNA binding but lacks cleavage activity; used for transcriptional regulation . |
V. Applications Leveraging Structural Features
1. Genome Editing
- Knockouts: sgRNA directs Cas9 to disrupt genes via non-homologous end joining (NHEJ) .
- Knock-Ins: Homology-directed repair (HDR) facilitated by sgRNA’s precise targeting .
2. Epigenetic Regulation
- CRISPRa/i: dCas9-sgRNA complexes recruit transcriptional activators (e.g., VP64) or repressors (e.g., KRAB) to modulate gene expression .
3. Diagnostic Tools
- SHERLOCK: Leverages sgRNA-Cas13a complexes for RNA virus detection via collateral cleavage of reporter probes .
VI. Challenges and Innovations
1. Off-Target Effects
- Mismatch Tolerance: Even 1–2 mismatches in the seed region can cause unintended edits. Solutions include high-fidelity Cas9 variants (e.g., HypaCas9) and paired nickases .
2. Delivery Optimization
- Chemical Synthesis vs. Expression: Synthetic sgRNAs offer immediate activity, while vector-expressed sgRNAs enable sustained editing .
3. AI-Driven Design
- Deep Learning Models: Tools like DeepSpCas9 predict sgRNA efficiency and specificity using sequence-structure relationships .
Conclusion
sgRNA exemplifies the fusion of natural RNA biology and synthetic engineering. Its modular architecture—combining a target-specific crRNA with a structural tracrRNA scaffold—enables unparalleled precision in genome editing. Advances in chemical modification, scaffold engineering, and AI-driven design continue to expand its applications in therapeutics, diagnostics, and functional genomics.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com




