Advancing Genome Engineering, Synthetic Biology, and Therapeutic Development
1. Genome Editing Tools for Stop Codon Replacement
A. Multiplex Automated Genome Engineering (MAGE)
Developed by Harvard researchers, MAGE enables precise, large-scale replacement of stop codons across entire genomes. This technology uses synthetic oligonucleotides to replace target codons in vivo. For example, in E. coli, all 314 TAG stop codons were replaced with TAA, eliminating redundancy and freeing TAG for non-canonical amino acid encoding. MAGE’s high-throughput capability allows simultaneous editing of multiple loci, accelerating synthetic genome design.
Image suggestion: Workflow of MAGE technology, illustrating oligonucleotide design, homology-directed repair, and genome-wide TAG-to-TAA replacement.
B. Conjugative Assembly Genome Engineering (CAGE)
Building on MAGE, CAGE merges edited genomic segments from distinct bacterial strains into a single organism. This method enabled the consolidation of 80 synonymous codon replacements in E. coli, including genome-wide TAG-to-TAA conversions, without synthetic lethality.
2. Stop Codon Suppression and Reassignment
A. Amber Suppression
Amber suppression repurposes the UAG (TAG) stop codon to encode non-standard amino acids (NSAAs). Key components include:
- Orthogonal tRNA/synthetase pairs: Engineered tRNA (e.g., with CUA anticodon) and aminoacyl-tRNA synthetases charge NSAAs like photocrosslinkers or fluorophores.
- Applications: Site-specific protein labeling, incorporation of bioactive molecules, and protein functional studies in mammalian cells.
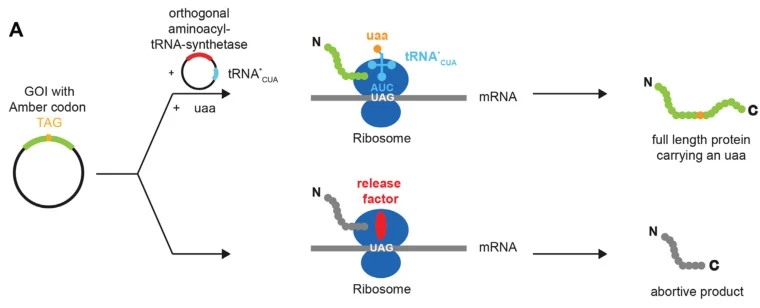
Image suggestion: Mechanism of amber suppression: orthogonal tRNA charging with NSAA and incorporation at UAG sites.
B. Quadruplet Codons
Expanding beyond triplet codons, quadruplet systems (e.g., 4-base codons) introduce novel termination signals. These systems reduce cross-talk with natural stop codons and enable orthogonal genetic codes for NSAAs.
3. Genomic Recoding and Codon Compression
A. Unifying Stop Signals
The E. coli “Ochre” strain exemplifies codon compression, where all stop codons (UAG, UAA, UGA) are replaced with UAA. This required:
- Engineering release factors: Modifying RF2 to reduce UGA recognition.
- tRNA adjustments: Reprogramming tRNATrp to prevent UGA misincorporation.
This freed UAG and UGA for NSAAs, enabling dual NSAA incorporation with >99% accuracy.
B. Synthetic Genome Construction
The Syn61 E. coli genome replaced 18,214 codons using whole-genome synthesis, reducing the genetic code to 61 codons. This included replacing TAG with TAA, demonstrating scalability in codon reassignment.
Image suggestion: Comparison of natural and Syn61 genomes, highlighting codon replacement density.
4. CRISPR-Cas9 and Base Editing
A. CRISPR-Mediated Replacement
CRISPR-Cas9 introduces stop codons into target genes for functional knockout (e.g., PD-1 in CAR-T cells) or corrects premature termination codons (PTCs) in diseases like cystic fibrosis.
B. Multiplex Base Editing
Recent advancements allow simultaneous conversion of TAG to TAA across multiple human genes. Tools like GRIT software optimize guide RNA design for efficient, off-target-free editing.
5. Therapeutic and Industrial Applications
A. Disease Modeling and Therapy
- Premature stop codon correction: Readthrough drugs (e.g., ataluren) or CRISPR editing restores functional protein expression in disorders like Duchenne muscular dystrophy.
- Resurrecting pseudogenes: Replacing inactivating stop codons in pseudogenes (e.g., human urate oxidase) revived enzymatic activity for therapeutic use.
B. Bioproduction Optimization
Recoded organisms with unified stop codons improve metabolic pathway efficiency. For example, TAA homogenization in E. coli enhanced recombinant protein yields by minimizing translational errors.
6. Challenges and Future Directions
A. Technical Hurdles
- Off-target effects: High-fidelity editors and AI-driven design tools (e.g., DeepCRISPR) mitigate unintended edits.
- Delivery limitations: Viral vectors or lipid nanoparticles must accommodate large editing systems for in vivo applications.
B. Ethical and Safety Considerations
- Biocontainment: Recoded organisms engineered with xenobiotic dependencies prevent environmental escape.
- Regulatory harmonization: Global standards are needed for clinical translation of codon-edited therapies.
C. Emerging Frontiers
- AI-optimized codon tables: Machine learning predicts codon compatibility for synthetic genomes.
- Expanded genetic codes: Integrating >100 NSAAs via compressed codon frameworks for novel biomaterials.
Proposed Figure Descriptions
- Figure 1: MAGE workflow for genome-wide TAG-to-TAA replacement in E. coli.
- Figure 2: Mechanism of amber suppression using orthogonal tRNA/synthetase pairs.
- Figure 3: Structural comparison of natural urate oxidase vs. pseudogene-resurrected enzyme with stop codon edits.
Data Source: Publicly available references.
Contact: chuanchuan810@gmail.com




