 I. Foundational Discoveries: The Pre-Recombinant Era (1869-1970)
I. Foundational Discoveries: The Pre-Recombinant Era (1869-1970)
The journey began with Friedrich Miescher’s 1869 isolation of DNA from salmon sperm, establishing nucleic acids as carriers of genetic information . Critical milestones followed:
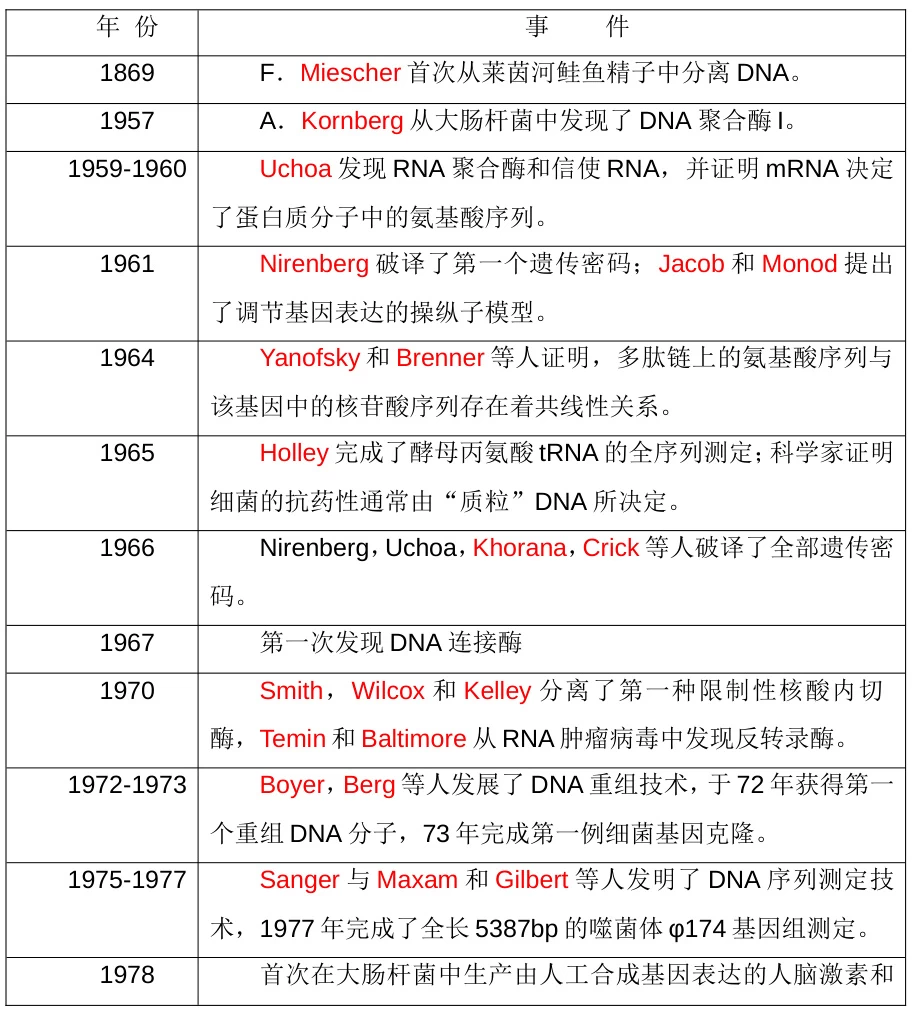
- 1953: Watson & Crick deciphered DNA’s double-helix structure, revealing molecular self-replication .
- 1967: Discovery of DNA ligase enabled enzymatic stitching of DNA fragments .
- 1970: Restriction endonucleases (e.g., EcoRI) were characterized by Smith, Wilcox & Kelley, providing precision “molecular scissors” .


(Fig. 1: Molecular Toolkit Development)
Description: DNA double helix (gold) with EcoRI (blue) cleaving GAATTC sequence. DNA ligase (green) stitches fragments.
II. Birth of Recombinant DNA Technology (1972-1973)
1972: Paul Berg’s team (Stanford) created the first recombinant DNA molecule by splicing SV40 virus DNA into λ phage DNA using EcoRI . This in vitro recombination proved genes could be transplanted across species.
1973: Cohen & Boyer’s landmark experiment :
- Cleaved E. coli plasmid pSC101 with EcoRI
- Inserted frog ribosomal DNA fragment
- Transformed recombinant plasmid into bacteria
- Demonstrated stable replication and expression
This established the core workflow: cut → splice → transform → express.
(Fig. 2: Cohen-Boyer Experiment)
Description: Plasmid (circular blue) and frog DNA (linear red) cleaved by EcoRI. Recombinant plasmid transformed into E. coli expressing new genes.
III. Technological Expansion (1975-1990s)
A. Enabling Tools
| Year | Innovation | Impact |
|---|---|---|
| 1977 | Sanger DNA sequencing | Gene characterization |
| 1983 | PCR (Mullis) | DNA amplification revolution |
| 1986 | Automated sequencers | High-throughput genomics |
B. Vector Systems Evolution
- Plasmids: pBR322 (1977) with antibiotic markers
- Bacteriophages: λ vectors for larger inserts
- Artificial Chromosomes: YACs/BACs for megabase DNA
C. First Commercial Applications
- 1978: Recombinant human insulin (Genentech)
- 1982: FDA approval of Humulin® (first rDNA drug)
- 1994: Flavr Savr™ tomato (first GMO food crop)
(Fig. 3: Therapeutic Milestones)
Description: Insulin vials, Bt-cotton fields, and DNA sequencing gels symbolizing medical/agricultural applications.
IV. Ethical Governance & Public Discourse
The 1975 Asilomar Conference established critical safeguards:
- Biological Containment: Disabled E. coli K12 strains
- Physical Containment: Biosafety Level guidelines
- Research Moratorium: On high-risk experiments until protocols finalized
“We were creating new genetic combinations that had never existed on Earth. Prudence was non-negotiable.”
— Paul Berg, Asilomar Proceedings
V. Modern Integration (2000-Present)
A. Convergence with Genomics
- Human Genome Project (2003): Enabled gene-targeted therapies
- CRISPR-Cas9 (2012): Precision editing integrated with rDNA workflows
B. Cutting-Edge Applications
| Field | Breakthrough |
|---|---|
| Medicine | CAR-T cell therapies (engineered receptors) |
| Agriculture | Golden Rice (β-carotene biofortification) |
| Environment | Pseudomonas strains degrading oil spills |
C. Global Regulatory Frameworks
- Cartagena Protocol (2003): International GMO biosafety standards
- FDA/EMA Guidelines: rDNA drug approval pathways (e.g., mRNA vaccines)
VI. Future Trajectories
A. Synthetic Biology Integration
- DNA Foundries: Automated gene assembly platforms
- Xenobiology: Artificial nucleotides expanding genetic alphabet
B. Emerging Challenges
- Equitable Access: Global South technology transfer
- Biosecurity: Preventing malicious use of DIY bio-labs
(Fig. 4: Next-Generation Genetic Engineering)
Description: Automated DNA synthesizer (left), CRISPR-Cas12 editing complex (center), 3D-printed synthetic cells (right).
“Recombinant DNA technology transformed biology from an observational science into a design discipline—giving us the tools to rewrite the code of life.”
— Nature Biotechnology, 2025
Conclusion: The Engineered Epoch
From Berg’s test tube to global biotechnology ecosystems, rDNA technology has:
- Democratized Genetic Manipulation: Cost per DNA base pair dropped from 10,000(1973)to0.01 (2025)
- Redefined Medicine: 40% of FDA-approved drugs now biologics produced via rDNA
- Reshaped Agriculture: GMO crops planted on 190 million hectares worldwide
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.




