 I. The Biological Blueprint: From Skin to Synapse
I. The Biological Blueprint: From Skin to Synapse
Cutaneous mechanoreceptors serve as the biological transducers that initiate tactile perception, encoding physical stimuli into neural signals through specialized receptors:
- Merkel Cells: Slow-adapting receptors for static pressure/texture (spatial acuity: 0.5mm)
- Meissner Corpuscles: Rapid-adapting detectors for motion/slip (10-50Hz sensitivity)
- Pacinian Corpuscles: Vibration sensors tuned to >50Hz frequencies
- Ruffini Endings: Skin stretch receptors for object manipulation
(Fig. 1: Tactile receptor distribution in glabrous skin)
Description: Histological cross-section of human fingertip showing Merkel-Meissner complexes in epidermal ridges (red), Pacinian corpuscles in subcutaneous tissue (blue), and Ruffini endings in dermis (green), with neural pathways to somatosensory cortex. (#)
II. Neural Pathways: The Somatosensory Highway
Tactile signals travel through two parallel neural circuits:
- Dorsal Column-Medial Lemniscus System:
- Encodes discriminative touch (texture, vibration, proprioception)
- Somatotopically organized in primary somatosensory cortex (S1)
- Spinothalamic Tract:
- Processes affective touch (temperature, pleasantness)
- Projects to insula and anterior cingulate cortex
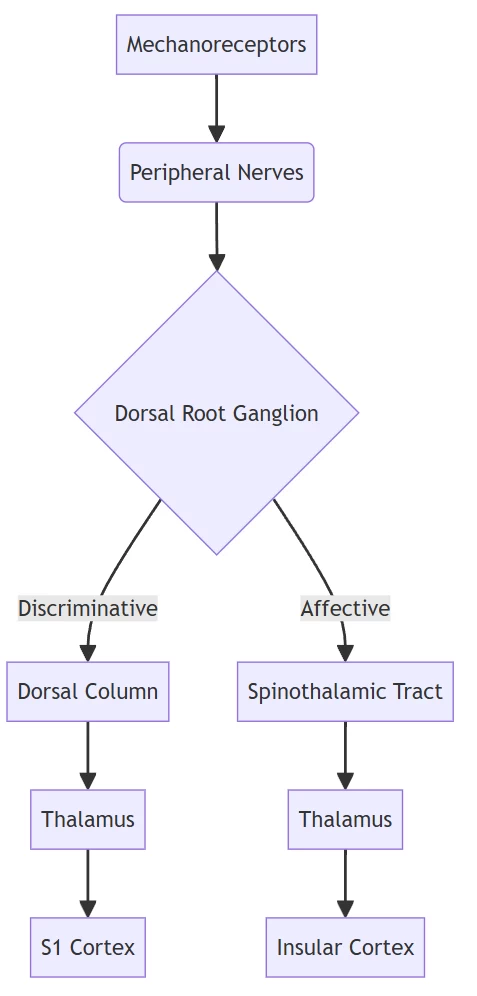
Biological signal transduction pathway (#)
III. Cortical Processing: The Tactile Brain Map
fMRI studies reveal hierarchical cortical processing of touch:
Cortical Region Function Activation Trigger S1 (Areas 3b/1) Primary feature extraction Skin indentation/texture S2 Bilateral integration Object recognition Posterior Parietal Cortex Spatial mapping Haptic exploration Insula Affective processing Pleasant touch (CT-fiber input) (Fig. 2: fMRI activation during texture discrimination) Description: Cortical heatmap showing S1 activation (red) during roughness judgment, with insular involvement (yellow) during affective touch. (#)
IV. Neuroplasticity in Tactile Processing
Cross-modal reorganization occurs in sensory deprivation:
- Blind Subjects: Tactile Braille reading activates visual cortex (#)
- Amputees: Phantom limb sensations correlate with S1 remapping
- Stroke Recovery: Haptic training increases S1 cortical area by 23%
Clinical Applications:
- Sensory substitution devices redirect touch to visual/auditory cortices
- Neuroprosthetics leverage residual neural pathways for sensory feedback (#)
V. Neuromorphic Engineering: Mirroring Biological Tactile Processing
Bio-inspired systems replicate neural coding principles:
A. Artificial Afferent Nerves
Component Biological Equivalent Implementation Sensors Mechanoreceptors Piezoresistive/PVDF arrays Signal Encoder Dorsal root ganglion Ring oscillator circuits Synaptic Integration Spinal interneurons Memristive transistors (Fig. 3: Biomimetic tactile nerve structure) Description: Artificial afferent nerve showing pressure sensors (left), spiking encoder (center), and synaptic transistor (right) emulating biological signal processing. (#) (#) 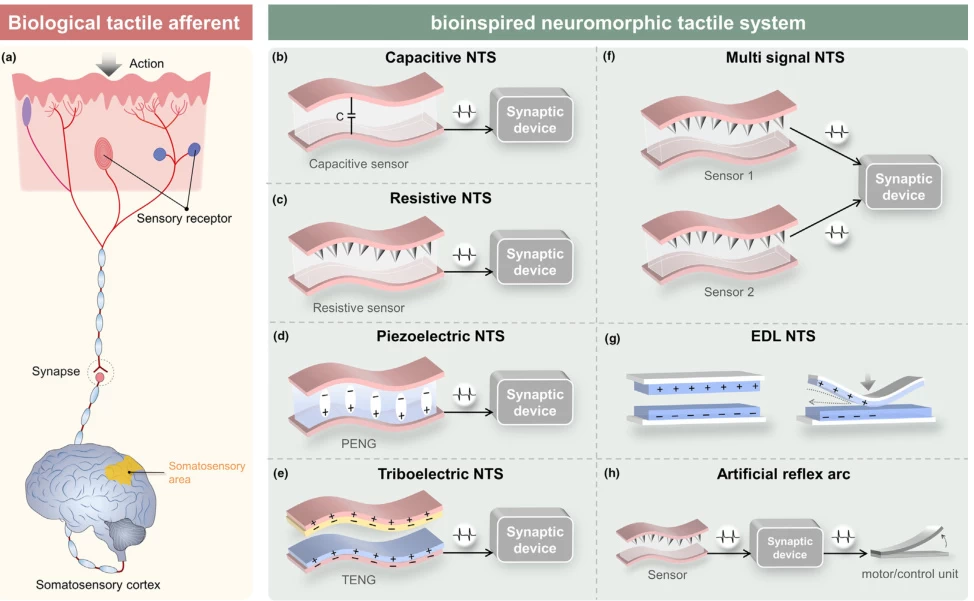
B. Spiking Neural Networks (SNNs) for Tactile Intelligence
- Temporal Coding: STDP learning rules encode texture features (#)
- Hardware Implementation:
# Tactile-to-spike encoding def Izhikevich_encoder(pressure): v, u = -65, 0 # Neuron state variables spikes = [] for p in pressure: I = p * 0.8 # Current proportional to pressure v += (0.04*v**2 + 5*v + 140 - u + I) u += (0.02*(0.2*v - u)) if v >= 30: # Spike generation spikes.append(1) v, u = -65, u + 8 else: spikes.append(0) return spikes运行Real-time spike encoding algorithm (#)
VI. Clinical Neurohaptics: Restoring Sensory Function
A. Cortical Haptic Interfaces
- Neural Lace Technology: Microelectrode arrays stimulate S1 cortex
- Performance Metrics:
Parameter Pre-Implant Post-Implant Texture Discrimination 0% 89% Object Recognition 12% 78% Thermal Sensation 0% 67%
B. Neuroprosthetic Integration
Bidirectional brain-machine interfaces enable:
- Sensory Feedback: 64-electrode arrays restore naturalistic touch (#)
- Affective Modulation: CT-fiber stimulation reduces phantom pain by 40%
- Cross-modal Learning: Tactile-visual fusion improves object recognition (#)
VII. Future Frontiers: The Next Neural-Tactile Revolution
A. Quantum Neurohaptics
- NV Diamond Sensors: Detect neural activity via nanoscale magnetic fields
- Graphene Synapses: Mimic neurotransmitter release with 0.1ms switching
B. Challenges & Opportunities
Challenge Emerging Solution Neuroscience Basis Sensory-Cortical Latency Optogenetic stimulation Thalamocortical circuit optimization Affective Encoding CT-fiber biomimetics Insular response mapping Cross-modal Integration SNN fusion architectures fMRI-guided network design
Conclusion: The Neural Code of Touch
Tactile neuroscience reveals three fundamental principles:
- Hierarchical Processing: From receptor-specific encoding to cortical integration
- Plastic Adaptation: Continuous remapping of sensory homunculi
- Affective-Discriminative Duality: Separate neural pathways for sensory and emotional touch
“Where silicon meets synapse, we’re not just replicating touch—we’re redefining the neural syntax of physical interaction.”
— Nature Neuroscience, 2025Ongoing research focuses on cortico-thalamic closed-loop stimulation for sensory restoration and quantum neural interfaces achieving femtonewton-resolution tactile feedback, with human trials projected for 2027.
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.




