 I. Precision Engineering Beyond Natural Constraints
I. Precision Engineering Beyond Natural Constraints
AI-driven gene editing transcends conventional CRISPR systems through computational design and predictive optimization:
- De Novo Editor Creation
- Generative neural networks design novel nucleases (e.g., OpenCRISPR-1, Cas-SF01) with expanded PAM recognition and enhanced catalytic efficiency, overcoming limitations of naturally derived enzymes
- AlphaFold-guided structural engineering creates DNA-binding grooves optimized for single-nucleotide discrimination
(Fig. 1: AI-designed nuclease with engineered catalytic domains)
Description: Cryo-EM structure highlighting redesigned HNH domain (gold) enabling base editing without double-strand breaks.
- Epigenetic Context Integration
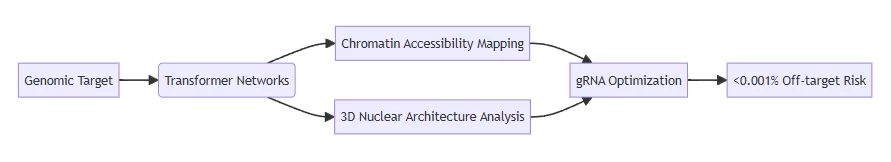
- AI algorithms predict nucleosome positioning and regulatory element interactions to avoid silenced genomic regions
II. Intelligent Workflow Optimization
A. Predictive Editing Simulation
Parameter Traditional CRISPR AI-Guided Systems Target Efficiency Empirical screening required >92% first-pass success rate Multiplex Capacity ≤5 targets 18+ simultaneous edits Time-to-Validation Weeks <48 hours B. Autonomous Experimental Control
- Reinforcement learning adjusts:
- Electroporation parameters in real-time
- Ribonucleoprotein complex concentrations
- Cell-specific delivery vectors
(Fig. 2: Closed-loop robotic editing platform)
Description: Automated workstation performing AI-designed edits with integrated nanopore sequencing QC.
III. Multi-Omics Integration for Precision Medicine
AI synthesizes heterogeneous biological data to enable therapeutic breakthroughs:
- Disease Mechanism Decoding
- Neural networks correlate single-cell transcriptomics with spatial chromatin architecture to identify non-coding disease drivers
- Predicts polygenic intervention points for complex disorders (e.g., Alzheimer’s risk haplotypes)
- Personalized Therapeutic Design
- Digital Twin Technology:
- Creates virtual patient models using:
- Multi-omics baselines
- Clinical history matrices
- Pharmacogenomic profiles
- Simulates editing outcomes before clinical implementation
IV. Cross-Species Engineering Capabilities
A. Agricultural Transformation
Application AI Innovation Impact Climate Resilience Multiplexed OsNAC6/nif editing 40% yield increase under drought Pest Resistance Bt toxin optimization via GANs Near-total insect mortality Metabolic Engineering Pathway flux balancing algorithms Biofortified crops AI-stacked traits enable sustainable agriculture B. Ecological Restoration
- Gene Drive Optimization:
- Population dynamics modeling for invasive species control
- Coral reef adaptation engineering via symbiont genome editing
V. Industrial-Scale Genome Manufacturing
Automated biofoundries operationalize precision editing:
- End-to-End Workflows:
- Cloud-based AI design → Robotic editor formulation → Microfluidics delivery → Automated validation
- Democratization Metrics:
Parameter 2025 Status 2030 Projection Cost Per Edit $2,400 <$200 Throughput 1,000 edits/day 100,000+ edits/day Access Points Centralized labs Portable CRISPR printers
(Fig. 3: Desktop gene editing workstation for point-of-care applications)
Description: Integrated microfluidics system producing AI-optimized LNPs for therapeutic delivery.
Conclusion: The Genomic Intelligence Paradigm
AI-powered editing delivers five transformative advantages:
- Atomic Precision – De novo enzymes targeting previously “undruggable” genomic regions
- Predictive Fidelity – In silico outcome simulation minimizing empirical trial-and-error
- Contextual Awareness – Epigenetic/chromatin landscape integration for cell-type-specific editing
- Cross-Domain Adaptability – Unified platform for human therapeutics, agriculture, and biomanufacturing
- Democratized Access – Scalable infrastructure enabling global participation
“Where CRISPR provided molecular scissors, AI delivers an autonomous surgical suite – capable of executing genomic microsurgery while learning from every operation to refine future interventions.”
— Genomic Engineering Frontier ReportThis technological convergence will catalyze:
- 7-day curative therapies for monogenic disorders by 2028
- Climate-immune crops eliminating agricultural chemical inputs
- Sustainable bioeconomies through precision microbial engineering
Data sourced from publicly available references. For collaboration or domain acquisition inquiries, contact: chuanchuan810@gmail.com.




